Merging photoredox with copper catalysis: enantioselective remote cyanation via 1,4-heteroaryl migration†
Abstract
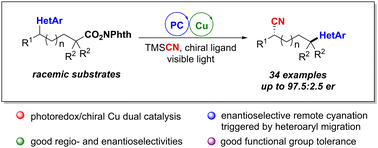
We have developed a photoredox/Cu dual catalyzed enantioselective remote cyanation via 1,4-heteroaryl migration. Experimental and computational studies have been carried out to reveal the reaction mechanism and explain the origins of the regio- and enantioselectivities of the remote cyanation process. This methodology exhibits mild conditions, a broad substrate scope and good regio- and enantioselectivities, which provides a unique approach for catalyst-controlled asymmetric backbone recombination of molecules via functional group migration.


 Please wait while we load your content...
Please wait while we load your content...