Asymmetric total synthesis of (+)-dihydroitomanallene B and formal synthesis of (−)-kumausallene†
Abstract
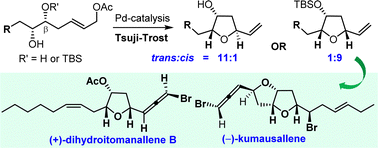
The first asymmetric total synthesis of (+)-dihydroitomanallene B and its two diastereomers, and the formal synthesis of (−)-kumausallene are reported. The synthesis of the former was completed in 18 steps from 1,4-butanediol (3.4% overall yield), with diastereoselective Tsuji–Trost cyclization to access cis-2,5-disubstituted-3-oxygenated THF scaffold and a Corey–White–Posner reaction to install the bromoallene moiety as the key steps. In addition, the enantioselective formal total synthesis of (−)-kumausallene involving the key Tsuji–Trost cyclization is also realized.


 Please wait while we load your content...
Please wait while we load your content...