Highly enantioselective synthesis of norcaradiene derivatives from naphthyl diazoacetamides using a Ru(ii)-Pheox complex†
Abstract
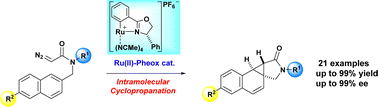
The highly regio- and enantioselective intramolecular cyclopropanation reactions of naphthyl diazoacetamides have been reported herein. In the presence of a Ru(II)-Pheox catalyst, chiral and stable norcaradiene derivatives were obtained from the corresponding diazoacetamides via carbene transfer reactions in high yields (up to 99%) and with high enantioselectivities (up to 99% ee).


 Please wait while we load your content...
Please wait while we load your content...