Chemical design of organic ferroelectrics using dynamics of alkylamide chains†
Abstract
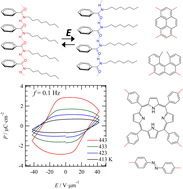
The polypeptide chain is an important structural unit that forms the secondary structure of proteins via intermolecular amide-type N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) hydrogen bonds. In contrast, alkylamide chains (–CONHCnH2n+1) are interesting structural units in which an amide group with a dipole moment and an alkyl chain with high conformational freedom coexist, which have been used to form characteristic molecular assembly structures such as liquid crystals, gels, and supramolecular polymers. One-dimensional chains based on intermolecular N–H⋯O
hydrogen bonds. In contrast, alkylamide chains (–CONHCnH2n+1) are interesting structural units in which an amide group with a dipole moment and an alkyl chain with high conformational freedom coexist, which have been used to form characteristic molecular assembly structures such as liquid crystals, gels, and supramolecular polymers. One-dimensional chains based on intermolecular N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) hydrogen-bonding interaction form these molecular assemblies. In the liquid crystal, an electric field – polarization hysteresis curve was observed for alkylamide-substituted benzene derivatives. A molecular design based on the combination of the alkylamide chain, which is the origin of ferroelectricity, and the functional π-electron framework enables the coexistence of ferroelectricity with luminescence, high thermal stability, photoresponse, and current switching properties, and the fabrication of multifunctional molecular materials. The introduction of chiral alkylamide chains is also effective in controlling unidirectional dipole inversion and low coercive electric fields for low-energy-consumption memory devices. Alkylamide chains in molecular assemblies are useful for the development and control of the physical properties of organic ferroelectrics that realize the rotational dynamics of polar amide groups coupled with the melting of alkyl chains in the liquid crystal and solid states.
hydrogen-bonding interaction form these molecular assemblies. In the liquid crystal, an electric field – polarization hysteresis curve was observed for alkylamide-substituted benzene derivatives. A molecular design based on the combination of the alkylamide chain, which is the origin of ferroelectricity, and the functional π-electron framework enables the coexistence of ferroelectricity with luminescence, high thermal stability, photoresponse, and current switching properties, and the fabrication of multifunctional molecular materials. The introduction of chiral alkylamide chains is also effective in controlling unidirectional dipole inversion and low coercive electric fields for low-energy-consumption memory devices. Alkylamide chains in molecular assemblies are useful for the development and control of the physical properties of organic ferroelectrics that realize the rotational dynamics of polar amide groups coupled with the melting of alkyl chains in the liquid crystal and solid states.


 Please wait while we load your content...
Please wait while we load your content...