N-Chlorobenzimidazoles as efficient and structurally diverse amphoteric halogen bond donors in crystal engineering†
Abstract
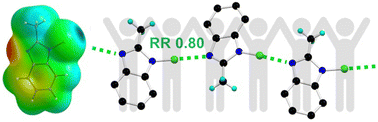
The ability of amphoteric N-chlorobenzimidazoles to self-associate into 1D chains through strong and linear N–Cl⋯N halogen bond interactions is demonstrated. The less polarisable Cl atom is strongly activated thanks to the intramolecular amphoteric character and the intermolecular cooperativity effect. The obtained family of compounds featuring different substitution patterns provides opportunities toward the elaboration of macroscopic polar structures.


 Please wait while we load your content...
Please wait while we load your content...