Enantioselective C–H bond functionalization of aromatic ketones with 1,6-enynes via photoredox/cobalt dual catalysis†‡
Abstract
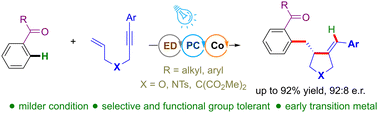
An enantioselective ortho-C(sp2)–H functionalization of ketones with 1,6-enynes is demonstrated via photoredox/cobalt dual catalysis. The method exhibits high yields, functional group tolerance, and selectivity. Mechanistic studies suggested the operation of visible-light mediated low-valent cobalt complex generation, intramolecular cyclization, ortho-C–H bond insertion, and reductive elimination as the key mechanistic steps.


 Please wait while we load your content...
Please wait while we load your content...