Last-step 18F-fluorination of supported 2-(aryl-di-tert-butylsilyl)-N-methyl-imidazole conjugates for applications in positron emission tomography†
Abstract
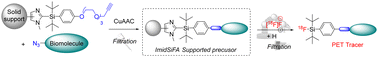
Aiming for potential applications in positron emission tomography, fully automated productions of 18F-labelled bioconjugates were achieved using heterogenous precursors obtained by anchoring imidazole-di-tert-butyl-arylsilanes to a polystyrene resin. The reactions were performed using either “batch” or “flow” procedures, avoiding both the time-consuming azeotropic drying and HPLC purifications usually required.


 Please wait while we load your content...
Please wait while we load your content...