Metal-free trans-hydroboration without a B–H bond: reactions of propargyl amines with Lewis acidic boranes†
Abstract
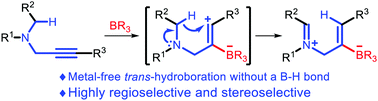
The first alkyne trans-hydroboration reaction without a B–H bond was described. This was achieved by reactions of propargyl amines with Lewis acidic boranes under mild conditions. The mechanism involved borane-mediated alkyne activation and intramolecular hydride transfer, thus realizing a unique hydroboration in the absence of a B–H bond.


 Please wait while we load your content...
Please wait while we load your content...