Synthesis of allylanilines via scandium-catalysed benzylic C(sp3)–H alkenylation with alkynes†
Abstract
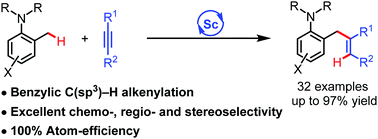
The ortho-selective benzylic C(sp3)–H alkenylation of 2-methyl tertiary anilines with internal alkynes has been achieved for the first time by using a half-sandwich scandium catalyst. This protocol provides a straightforward route for the synthesis of a new family of 2-allylaniline derivatives, featuring broad substrate scope, 100% atom-efficiency, high yields, and high chemo-, regio-, and stereoselectivity.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...