Catalytic asymmetric inverse-electron-demand aza-Diels–Alder reaction of 1,3-diazadienes with 3-vinylindoles†
Abstract
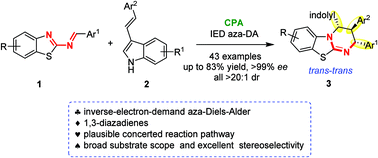
A facile chiral phosphoric-acid catalyzed asymmetric inverse-electron-demand aza-Diels–Alder reaction of 1,3-diazadienes with 3-vinylindoles was established. By using this mild and practical protocol, a broad range of benzothiazolopyrimidines with three contiguous stereogenic centers were prepared in good yields and excellent diastereo- and enantio-selectivities (43 examples, up to 83% yield, >99% ee and all >20 : 1 dr). A plausible concerted reaction pathway enabled by the dual hydrogen-bonding effect was proposed to account for the observed excellent enantioselectivity and specific trans–trans diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...