Diverse reactivity of an Al(i)-centred anion towards ketones†
Abstract
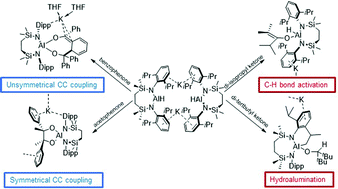
The reactivity of a seven-membered cyclic potassium diamidoalumanyl toward a variety of ketone small molecules has been assessed. Whilst acetophenone generates an aluminium pinacolate derivative, reductive C–C coupling is induced between the ketyl and ortho-carbon centres of two equivalents of benzophenone. In contrast, whereas oxidative addition of an enolisable proton is observed with 2,4-dimethyl-3-pentanone, 2,2,4,4-tetramethyl-3-pentanone undergoes an unprecedented hydroalumination process, where the reducing hydride may be traced to intramolecular oxidative addition of a (sp3)C–H bond.


 Please wait while we load your content...
Please wait while we load your content...