A base-free copper-assisted synthesis of C2-symmetric spirotelluranes and biaryls based on divergent stoichiometry of Na2Te†
Abstract
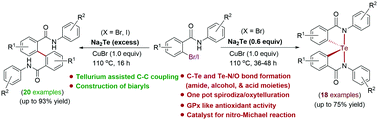
A one pot Cu(I)-assisted synthetic methodology has been developed for the preparation of biologically important C2-symmetric spirodiaza, benzyloxy and benzoxytelluranes from 2-bromo-N-aryl benzamides, benzyl alcohols, and benzoic acids by using the tellurium dianion (Te2−) under base-free conditions. Furthermore, C–C coupled biaryl 1,1′-diamides have been prepared by using an excess of Na2Te under the same reaction conditions. The synthesized spirodiazatelluranes served as a potent catalyst for the reduction of H2O2 and nitro-Michael reactions.


 Please wait while we load your content...
Please wait while we load your content...