A boron dipyrromethene chiral at boron and carbon with a bent geometry: synthesis, resolution and chiroptical properties†
Abstract
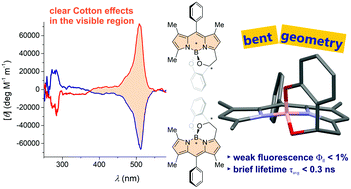
We report a boron dipyrromethene that is chiral at boron and carbon (B*C*-BODIPY) and accessible through a two-pot, one-step synthesis—an interrupted Knoevenagel condensation. The electronic circular dichroism spectra of chiral high performance liquid chromatography-resolved enantiomers show clear Cotton effects (∣gabs∣ ∼ 2.0 × 10−4) in the visible region, suggesting efficient chirality induction to the otherwise achiral BODIPY. The dye's unusually weak fluorescence (Φfl < 0.01) is attributed partly to vibrational relaxations, as revealed by viscosity experiments, and partly to probable intersystem crossing that may be facilitated by the reduced symmetry of the bent-shaped molecular geometry.


 Please wait while we load your content...
Please wait while we load your content...