Computational study revealed a “pull–push” radical transfer mechanism of Mmp10-catalyzed Cδ-methylation of arginine†
Abstract
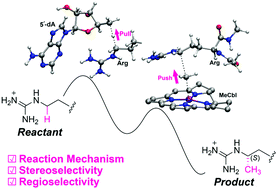
Mmp10 is a B12-dependent SAM radical enzyme that catalyzes Cδ-methylation of arginine. The quantum chemical cluster calculations of Mmp10 revealed a “pull–push” radical transfer mechanism in which a 5′-deoxyadenosine radical first abstracts a hydrogen atom from the arginine residue and then methylcobalamin donates a methyl group to the arginine residue. The stereoselectivity and regioselectivity of Mmp10 originated in a specific substrate binding mode enabled by the active site.


 Please wait while we load your content...
Please wait while we load your content...