Access to hexahydroazepinone heterocycles via palladium-catalysed C(sp3)–H alkenylation/ring-opening of cyclopropanes†
Abstract
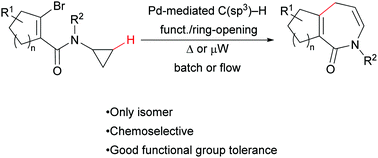
In this communication, we describe the synthesis of novel hexahydroazepinone derivatives starting from two simple building blocks in presence of a readily available palladium catalyst. The reaction proceeds through a selective C(sp3)–H alkenylation/ring-opening process to obtain the seven-membered ring products in good to excellent yields on a wide variety of substrates under batch, microwave, and continuous flow conditions.


 Please wait while we load your content...
Please wait while we load your content...