Facile C–H bond activation on a transient gallium imide†
Abstract
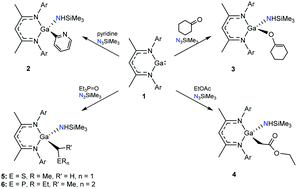
Reaction of NacNacGa with azide N3SiMe3 results in the generation of a transient imide NacNacGa(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSiMe3) that can cleave unactivated sp3 C–H and sp2 C–H bonds of different substrates, affording gallium amides. Pyridine, cyclohexanone, ethyl acetate, DMSO, and triethylphosphine oxide were activated in this process producing corresponding gallium amides. All new compounds were characterised by multinuclear NMR and X-ray diffraction.
NSiMe3) that can cleave unactivated sp3 C–H and sp2 C–H bonds of different substrates, affording gallium amides. Pyridine, cyclohexanone, ethyl acetate, DMSO, and triethylphosphine oxide were activated in this process producing corresponding gallium amides. All new compounds were characterised by multinuclear NMR and X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...