Diastereoselective addition of redox active esters to azomethine imines by electrosynthesis†
Abstract
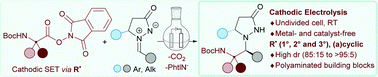
Thanks to metal- and catalyst-free electrochemical conditions in an undivided cell, a series of readily available redox-active N-(acyloxy)phthalimide esters led to an efficient and highly stereoselective addition (85 : 15 to 95 : 5 dr) of putative radical species to chiral (racemic and enantioenriched) C5-substituted azomethine imines to provide an array of 31 polyaminated hydrazine derivatives as a single diastereoisomer.


 Please wait while we load your content...
Please wait while we load your content...