In situ spectroscopic studies of the effect of water on the redox cycle of Cu ions in Cu-SSZ-13 during selective catalytic reduction of NOx†
Abstract
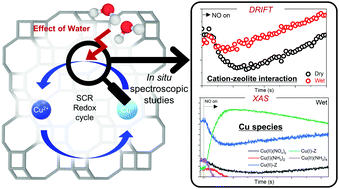
The effect of water on the NH3-assisted selective catalytic reduction of NOx (NH3-SCR) has been largely neglected, despite the inevitable presence of water vapor in real emissions produced by fuel combustion. In this work, we investigated the role of water in the behavior of active Cu2+ ions in Cu-SSZ-13 in the NH3-SCR reaction. The addition of water to the reactant feed leads to significantly increased NOx reduction over the catalyst. By combining in situ DRIFTS and XANES analyses during the NH3-SCR reaction, we found that the redox cycle of Cu ions is promoted by the presence of water.


 Please wait while we load your content...
Please wait while we load your content...