Asymmetric total synthesis of prostaglandin C2 TBS ether†
Abstract
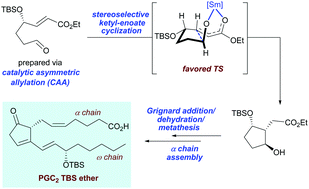
We disclose an asymmetric total synthesis of prostaglandin C2 TBS ether, a derivative of an extremely sensitive natural prostaglandin C2. The key to the synthesis is a SmI2-mediated ketyl–enoate reaction that leads to the formation of the functionalized cyclopentane ring with high-level stereochemical control. Access to the crucial alkene system is realized late in the synthesis by the implementation of a Grignard addition/dehydration/metathesis sequence.


 Please wait while we load your content...
Please wait while we load your content...