Enantioselective Pd-catalyzed dearomative reductive Heck and domino Heck–Suzuki reactions of 2-CF3-indoles†
Abstract
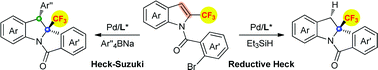
Highly enantioselective palladium-catalyzed dearomative reductive Heck reaction and domino Heck–Suzuki reaction of 2-CF3-indoles have been developed. Using Pd(OAc)2/(R)-Synphos as the catalyst and Et3SiH as a hydride source, a variety of indolines bearing a 2-trifluoromethyl quaternary stereocenter were obtained via a dearomative reductive Heck reaction. Alternatively, using Pd(dba)2/phosphoramidite as the catalyst and Ar4BNa as a coupling partner, structurally diverse indolines containing two vicinal carbon stereocenters were afforded through the domino dearomative Heck–Suzuki reaction.


 Please wait while we load your content...
Please wait while we load your content...