Rapid access to 9-arylfluorene and spirobifluorene through Pd-catalysed C–H arylation/deaminative annulation†
Abstract
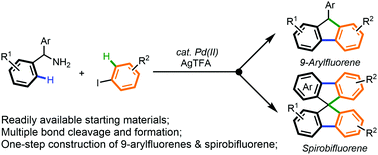
We describe here a facile synthesis of 9-arylfluorenes and spirobifluorenes from readily available 1,1-diarylmethylamines and iodoarenes through Pd-cataylsed C(sp2)–H arylation and a sequential deaminative annulation. The reaction features high efficiency and simplicity of operation, constituting an interesting shortcut to access fluorene compounds.


 Please wait while we load your content...
Please wait while we load your content...