Concise total syntheses of bis(cyclotryptamine) alkaloids via thio-urea catalyzed one-pot sequential Michael addition†
Abstract
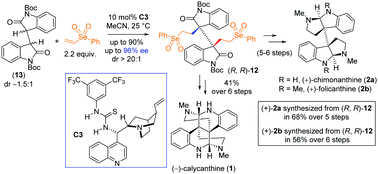
Naturally occurring bis(cyclotryptamine) alkaloids feature vicinal all-carbon quaternary stereocenters with an elongated labile C-3a–C-3a′ Sigma bond with impressive biological activities. In this report, we have developed a thio-urea catalyzed one-pot sequential Michael addition of bis-oxindole onto selenone to access enantioenriched dimeric 2-oxindoles with vicinal quaternary stereogenic centers at the pseudobenzylic position (up to 96% ee and >20 : 1 dr). This strategy has been successfully applied for the total syntheses of either enantiomers of chimonanthine, folicanthine, and calycanthine.


 Please wait while we load your content...
Please wait while we load your content...