Total syntheses of strained polycyclic terpenes†
Abstract
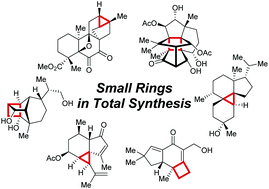
Terpenoids constitute a broad class of natural compounds with tremendous variability in structure and bioactivity, which resulted in a strong interest of the chemical community to this class of natural products over the last 150 years. The presence of strained small rings renders the terpenoid targets interesting for chemical synthesis, due to limited number of available methods and stability issues. In this feature article, a number of recent examples of total syntheses of terpenoids with complex carbon frameworks featuring small rings are discussed. Specific emphasis is given to the new developments in strategical and tactical approaches to construction of such systems.


 Please wait while we load your content...
Please wait while we load your content...