Straightforward synthesis of bench-stable heteroatom-centered difluoromethylated entities via controlled nucleophilic transfer from activated TMSCHF2†
Abstract
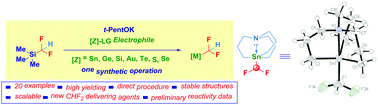
The commercially available and experimentally convenient (bp 65 °C) difluoromethyltrimethylsilane (TMSCHF2) is proposed as a valuable difluoromethylating transfer reagent for delivering the CHF2 moiety to various heteroatom-based electrophiles. Upon activation with an alkoxide, a conceptually intuitive nucleophilic displacement directly furnishes in high yields the bench-stable analogues.


 Please wait while we load your content...
Please wait while we load your content...