Site-selective C5–H and N–H alkylation of unprotected 8-aminoquinolines†
Abstract
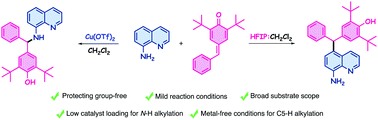
8-Aminoquinolines are the building blocks of many pharmaceutical compounds, which has motivated the scientific community to develop new ways to derivatize these compounds. In this work, we performed a site-selective C5–H and N–H alkylation of 8-aminoquinolines using para-quinone methides under extremely mild conditions. C5–H alkylation was performed using protecting group-free 8-aminoquinolines and in metal-free conditions. N–H alkylations were also carried out under mild conditions. All corresponding alkylation products were obtained in high to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...