Recent advances in difunctionalization of alkenes using pyridinium salts as radical precursors
Abstract
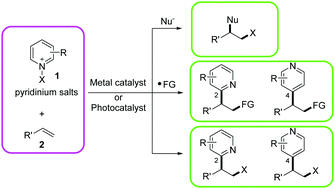
In this review, we summarise the recent applications of pyridinium salts in the radical-mediated difunctionalization of alkenes. Pyridinium salts are a privileged class of compounds that show great utility in natural products and synthetic chemistry. Various organic transformations of pyridinium salts, especially in radical chemistry, have been developed in recent years. We prepared this review based on the two distinguished properties of pyridinium salts in radical transformation: (1) pyridinium salts can easily undergo single electron reduction to deliver X radicals. (2) Pyridinium salts are highly electrophilic so that alkyl radical intermediates can easily add to the pyridine core. Based on the role of pyridinium salts in difunctionalization of alkenes, the main body of this review is divided into three parts: (1) using pyridinium salts as X transfer reagents. (2) Using pyridinium salts as novel pyridine transfer reagents. (3) Using pyridinium salts as bifunctional reagents (X and pyridine). The C2 and C4 selectivity during pyridylation is discussed in detail. We hope that this review will provide a comprehensive overview of this topic and promote the wider development and application of pyridinium salts.


 Please wait while we load your content...
Please wait while we load your content...