Rationally designed helical peptidomimetics disrupt α-synuclein fibrillation†
Abstract
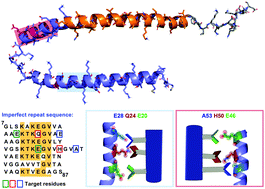
Misfolding of the human protein α-synuclein results in toxic fibrils and the aggregation of Lewy bodies, which are a hallmark of Parkinson's disease in brain tissue. Here we disclose a supramolecular approach where peptidomimetics are rationally designed and pre-organised to recognize the surface of native helical α-Syn by forming complementary contacts with key patches of protein surface composed of charged and hydrophobic residues. Under lipid-catalyzed conditions the mimetics slow the rate of aggregation (thioflavin-T assay) and disrupt the misfolding pathway (electron microscopy of aggregates). This hypothesis is supported by comparison with a series of negative control compounds and with circular dichroism spectroscopy. Given the approach relies on selective recognition of both amino acid sequence and conformation (helical secondary structure) there is potential to develop these compounds as tools to unravel the currently intractable structure–function relationships of (i) missense mutation, and (ii) amyloid polymorphism with disease pathogenesis.


 Please wait while we load your content...
Please wait while we load your content...