A novel cyclopentyl methyl ether electrolyte solvent with a unique solvation structure for subzero (−40 °C) lithium-ion batteries†
Abstract
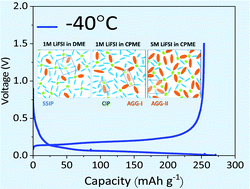
1 M LiFSI in cyclopentyl methyl ether is shown as a novel electrolyte with a unique solvation structure to form a thin robust multilayer solid electrolyte interface with an inorganic LiF-rich inner layer. Aggregates and contact ion pairs are actively formed in the solvation shell and reduced on the graphite anode during lithiation. This EC-free electrolyte provides 86.9% initial efficiency, and 355 mA h g−1 over 350 cycles with an excellent capacity retention of 84% at a 1C rate. An excellent low-temperature performance of 370, 337, and 330 mA h g−1 at 0, −10, and −20 °C, respectively, at a 0.1C rate is recorded. Furthermore, at −40 °C, the graphite half-cell has a capacity of 274 mA h g−1 without electrolyte freezing.


 Please wait while we load your content...
Please wait while we load your content...