Merging C–C σ-bond activation of cyclobutanones with CO2 fixation via Ni-catalysis†
Abstract
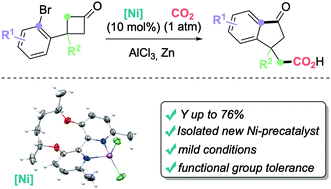
A carboxylative Ni-catalyzed tandem C–C σ-bond activation of cyclobutanones followed by CO2-electrophilic trapping is documented as a direct route to synthetically valuable 3-indanone-1-acetic acids. The protocol shows an adequate functional group tolerance and useful chemical outcomes (yield up to 76%) when AlCl3 is adopted as an additive. Manipulations of the targeted cyclic scaffolds and a mechanistic proposal based on experimental evidence complete the investigation.


 Please wait while we load your content...
Please wait while we load your content...