Fe–N–C/Fe nanoparticle composite catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells†
Abstract
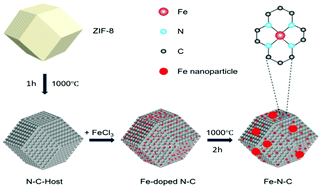
Replacing Pt-based catalysts with cost-effective, highly efficient, and durable platinum group metal-free catalysts for the oxygen reduction reaction (ORR) is crucial for commercializing hydrogen fuel cells. Herein, we present a highly active Fe–N–C electrocatalyst that contains both Fe nanoparticles and FeNx active sites derived from an Fe-doped carbonized zeolitic imidazolate framework (ZIF-8). It is found that adjusting the doping amount of Fe in the Fe-doped ZIF-8 precursor alters the morphology of the catalyst after heat treatment. The Fe–N–C-300 composite catalyst with the optimized Fe doping amount exhibits excellent activity, good stability, and remarkable methanol tolerance in the challenging acid environment. This study reveals that a suitable amount of Fe nanoparticles in the catalyst can alter the structure of the FeNx active moieties and increase three-phase boundaries to boost the mass transport, thus leading to improved fuel cell performance. This will have implications for using Fe–N–C catalysts in real applications, as the formation of Fe NPs during the synthesis and reaction is almost inevitable.


 Please wait while we load your content...
Please wait while we load your content...