Chirality-directed hydrogel assembly and interactions with enantiomers of an active pharmaceutical ingredient†
Abstract
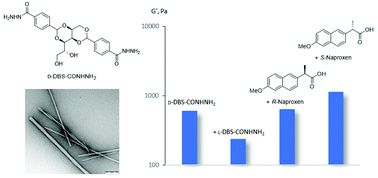
Enantiomers of the low-molecular-weight gelator (LMWG) DBS-CONHNH2, based on D- or L- 1,3 : 2,4-dibenzylidenesorbitol (DBS), were synthesised. Enantiomeric gels are equivalent, but when mixtures of enantiomers are used, although gels still form, they are weaker than homochiral gels. Nanoscale chirality is lost on adding even a small proportion of the opposite enantiomer – homochiral assembly underpins effective gelation. Enantiomeric gels encapsulate the two enantiomers of anti-inflammatory drug naproxen, with thermal & mechanical differences between diastereomeric systems. We hence demonstrate the importance of chirality in DBS assembly and its interactions with chiral additives.


 Please wait while we load your content...
Please wait while we load your content...