Photocaged dicarbonyl probe provides spatiotemporal control over protein glycation†
Abstract
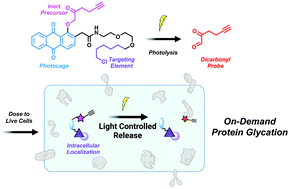
Protein glycation is a disease associated, non-enzymatic, posttranslational modification generated by endogenous dicarbonyl metabolites. Currently, there is a lack of chemical tools capable of studying protein adducts caused by this class of reactive species. Here, we report a chemical biology platform, termed T-DiP (targetable-dicarbonyl precursor), that releases a physiologically relevant dose of bio-orthogonally functionalized dicarbonyl probe upon irradiation with 365 nm light. This approach enables protein glycation to be controlled with spatiotemporal precision within live cells and expands the chemical toolbox needed to elucidate the roles of glycated proteins across various pathologies.


 Please wait while we load your content...
Please wait while we load your content...