Synthesis and modification of polymers by thiol-phenylsulfone substitution reaction†
Abstract
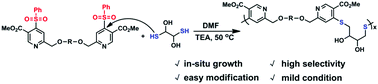
Thiol chemistry is a type of highly efficient chemical reaction between thiols and functional groups. During the past two decades, thiol chemistry has been widely applied in the synthesis and modification of polymers. With the rapid development of polymer chemistry and materials science, more thiol click reactions, which can be efficiently performed under mild conditions, are required. In this research, the synthesis and modification of polymers by thiol-phenylsulfone substitution reactions are reported. A monomer containing two phenylsulfonyl groups is synthesized and the monomer is reacted with bisthiols under mild conditions, leading to the synthesis of novel polymers. Size exclusion chromatography, 1H NMR and differential scanning calorimetry results demonstrate the step-growth polymerization of the monomer. A combination of thiol-phenylsulfone and thiol-disulfide reactions are used in the post-polymerization modification.


 Please wait while we load your content...
Please wait while we load your content...