Room-temperature Pd-catalyzed methoxycarbonylation of terminal alkynes with high branched selectivity enabled by bisphosphine-picolinamide ligand†
Abstract
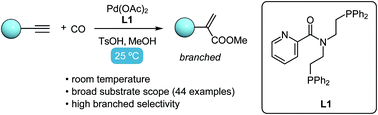
We report the room-temperature Pd-catalyzed methoxy-carbonylation with high branched selectivity using a new class of bisphosphine-picolinamide ligands. Systematic optimization of ligand structures and reaction conditions revealed the significance of both the picolinamide and bisphosphine groups in the ligand backbone. This strategic design of ligand was leveraged to deliver various α-substituted acrylates in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...