Ester-directed orthogonal dual C–H activation and ortho aryl C–H alkenylation via distal weak coordination†
Abstract
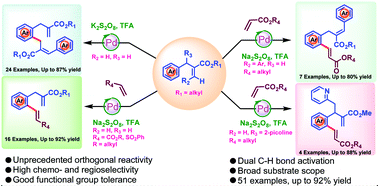
An unprecedented orthogonal cross-coupling between aromatic C(sp2) and aliphatic olefinic C(sp2) carbons of two same molecules via dual C–H bond activation in an intermolecular fashion has been developed using a distal ester-directing group. This new coupling reaction led to the synthesis of the highly functionalized 1,3-diaryl molecular architecture in very good yields and with high chemo- and regioselectivities. In addition, using ester as the distal directing group, ortho C–H olefination of α-methyl aryl acrylates and cinnamic esters with various alkenes has been achieved in very good yields and with a wide range of substrate scope.


 Please wait while we load your content...
Please wait while we load your content...