Canavanine utilization via homoserine and hydroxyguanidine by a PLP-dependent γ-lyase in Pseudomonadaceae and Rhizobiales†
Abstract
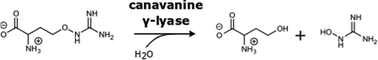
Canavanine, the δ-oxa-analogue of arginine, is produced as one of the main nitrogen storage compounds in legume seeds and has repellent properties. Its toxicity originates from incorporation into proteins as well as arginase-mediated hydrolysis to canaline that forms stable oximes with carbonyls. So far no pathway or enzyme has been identified acting specifically on canavanine. Here we report the characterization of a novel PLP-dependent enzyme, canavanine-γ-lyase, that catalyzes the elimination of hydroxyguanidine from canavanine to subsequently yield homoserine. Homoserine-dehydrogenase, aspartate–semialdehyde–dehydrogenase and ammonium–aspartate–lyase activities are also induced for facilitating canavanine utilization. We demonstrate that this novel pathway is found in certain Pseudomonas species and the Rhizobiales symbionts of legumes. The findings broaden the diverse reactions that the versatile class of PLP-dependent enzymes is able to catalyze. Since canavanine utilization is found prominently in root-associated bacteria, it could have important implications for the establishment and maintenance of the legume rhizosphere.


 Please wait while we load your content...
Please wait while we load your content...