Self-assembled di- and tripeptide gels for the passive entrapment and pH-responsive, sustained release of an antidiabetic drug, glimepiride†
Abstract
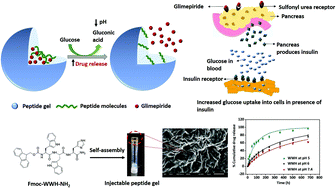
Diabetes is a global epidemic that poses a severe challenge to public health. The characteristic features of this disease are hyperglycemia and deterioration of the function of pancreatic β-cells, which leads to oxidative stress and organ damage. Glimepiride is used to treat type II diabetes but is associated with side effects, like lower half-life, faster elimination, and hypoglycemia. Self-assembled peptide gels have drawn attention as a drug delivery depot because of their biocompatibility, diverse design, tunable functionality, and dynamic self-assembly properties. In order to overcome the challenge of oxidative stress and side effects associated with the use of glimepiride, we have developed glimepiride-loaded, self-assembled peptide gels from di- and tripeptides employing amino acids with inherent antioxidant properties. Dipeptides, Fmoc-Tyr-Tyr-NH2 (YY) and Fmoc-Trp-Trp-NH2 (WW), and a tripeptide, Fmoc-Trp-Trp-His-NH2 (WWH), were developed and self-assembled into gels. The gels exhibited excellent viscoelastic properties and self-healing abilities, and the presence of β-sheet secondary structures. The dipeptide gels provided a sustained drug release but more drug was released at physiological pH (7.4) than acidic pH (5 and 6), whereas the tripeptide gel released more drug at acidic pH. The gels showed free radical scavenging activities of more than 90% and were able to decrease the amount of oxidative stress caused by the ROS in HepG2 cells. They were non-toxic to the cell line tested and HepG2 cells treated with the releasate of tripeptide gels showed enhanced glucose uptake. This work for the first time reports the development of glimepiride-loaded self-assembled peptide gels, which can serve as a dynamic, multidimensional biomaterial to reduce oxidative stress, hypoglycemia, and repetitive dosing of drugs in diabetic patients by controlling glimepiride release.


 Please wait while we load your content...
Please wait while we load your content...