In situ self-assembly of polydopamine inside injectable hydrogels: antibacterial activity and photothermal therapy for superbug-infected wound healing†
Abstract
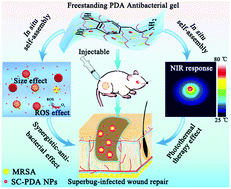
Inspired by the mussel foot proteins, polydopamine nanoparticles (PDA NPs) are often used to design hydrogel wound dressings due to their strong wet adhesion. However, additional antibiotics or nanometal bactericides are always required to enhance their poor antibacterial activity, which will cause drug resistance and toxic side effects. Herein, self-assembly confined PDA NPs (SC-PDA NPs, <50 nm) are employed as a freestanding antibacterial ingredient for constructing an ideal hydrogel wound dressing, which maintains relatively strong reducibility and size effect. Through a rapid gelation (within 10 s) strategy triggered by electrostatic complexation, an antibacterial hydrogel system is obtained, in which the in situ self-assembly of the SC-PDA NPs continues, endowing the gel with outstanding antibacterial activity, especially against methicillin-resistant Staphylococcus aureus (MRSA, >99.9%). With the continuous in situ self-assembly, the size of the PDA NPs increases (>200 nm), eventually giving the gel an efficient photothermal therapy effect. Moreover, the gel presents a relatively strong wet adhesion (63 kPa), superior biocompatibility and non-immunogenicity. This work offers innovative insights into the antibacterial mechanism of SC-PDA NPs and provides a novel design for constructing safe antibacterial hydrogel wound dressings, demonstrating great potential applications in superbug-infected wound healing.


 Please wait while we load your content...
Please wait while we load your content...