Rational engineering and synthetic applications of a high specificity BiFC probe derived from Springgreen-M†
Abstract
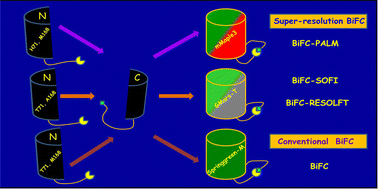
As an indispensable genetically encoded optical method for detecting and visualizing protein–protein interactions (PPIs) directly in live cells, bimolecular fluorescence complementation (BiFC) assay has gained popularity over the past decade mainly because of its high sensitivity and easy usage. However, most existing fluorescent protein-based BiFC (FP-BiFC) assays still suffer from relatively low specificity or imaging signal-to-noise (S/N) ratios. Thus, developing high S/N ratio BiFC probes, especially in the widely used bright green-yellow region of the spectrum is very meaningful. In addition, synthetic engineering of BiFC probes which can be readily used for multiplexing imaging is also highly valuable for uncovering more or new layers of information on PPIs. In this report, we developed a bright stable green fluorescent protein Springgreen-M based on our previously evolved fast reversible photoswitching fluorescent protein (RSFP) GMars-T. We then established a novel BiFC assay based on Springgreen-M for imaging PPIs in live cells with high specificity. Combined with the same lineage, BiFC assays readily developed from photoconvertible fluorescent protein mMaple3 or reversibly photoswitchable fluorescent protein GMars-T, high specificity multiplexing imaging of PPIs could also be realized in the same live cell. Thus, our newly developed Springgreen-M and Springgreen-M-based BiFC probes will meet the urgent need for high-specificity BiFC detection, flexible visualization and screening of PPIs in live cells.


 Please wait while we load your content...
Please wait while we load your content...