Pressure-induced chemical decomposition of copper orthovanadate (α-Cu3V2O8)†
Abstract
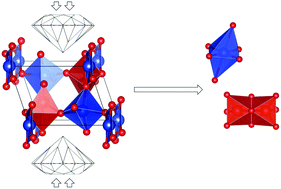
The high pressure stability of α-Cu3V2O8 has been investigated via complementary high pressure synchrotron X-ray diffraction experiments and theoretical density functional theory calculations. The results of both experiment and theory are in close agreement. The main result of this work is that α-Cu3V2O8 undergoes a pressure-induced chemical decomposition into CuO and V2O5 at a modest pressure of ∼1.35 GPa according to the experimental observations, and at ∼2.45 GPa according to the calculations. The decomposition is investigated with enthalpy calculations and one of the main driving factors is the stability of the octhedral oxygen-coordination of the metal atoms in the decompositon products. Additionally, we determine the bulk modulus of α-Cu3V2O8 to be the lowest of all known vanadates, at 52(2) GPa, due to its crystal structure, and determine the corresponding isothermal compressibility tensor.


 Please wait while we load your content...
Please wait while we load your content...