Physical insights from the Frumkin isotherm applied to electrolyte gated organic transistors as protein biosensors†
Abstract
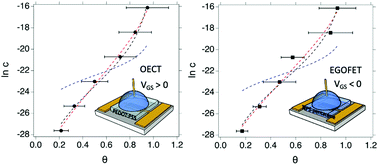
Label free biosensors based on electrolyte gated organic transistors (EGOTs) are ultra-sensitive and versatile sensing devices. The dose curve represents the change of the sensor signal as a function of the concentration of the target analyte, and, under the hypothesis of dynamic equilibrium between the surface-bound probe and its target partner, can be fitted to adsorption isotherms. In this work, we show that the data obtained from both the OECT and EGOFET Interleukin-6 (IL-6) biosensors are best fitted by the Frumkin isotherm compared to the widely adopted Langmuir and Hill isotherms. Comparable values of the equilibrium association constant Ka and the Frumkin interaction parameter g′ are obtained with both OECT and EGOFET sharing the same functionalization of the gate electrode. Our study unambiguously shows that the biosensor response is, to a large extent, due to the specific binding at the gate/electrolyte interface, and that is viable to investigate the thermodynamics of biorecognition. Moreover, the electrostatic repulsions between adsorbed probe–target pairs are shown to decrease the effective equilibrium association constant as coverage increases, thus causing a loss of sensitivity for concentrations above the threshold limit 1/|g′|.
- This article is part of the themed collection: Materials for molecular electronics and magnetism


 Please wait while we load your content...
Please wait while we load your content...