A novel phosphor of Cu+-doped PbBrOH: preparation, luminescence mechanism, and outstanding properties
Abstract
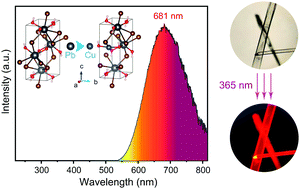
In this study, for the first time, a Cu+-doped PbBrOH red phosphor (PbBrOH:Cu) was successfully synthesized by a hydrothermal method. The crystal structure, microstructure, optical properties, luminescence mechanism, and environmental stability of the PbBrOH:Cu phosphor were discussed in detail. PbBrOH and PbBrOH:Cu phosphor synthesized by the hydrothermal method are needle-like transparent grains. The excitation wavelengths of the PbBrOH and PbBrOH:Cu phosphor were both 353 nm. Under the excitation of this wavelength, weak blue emission and excellent red emission were observed. Based on first-principles calculation, ultraviolet and visible (UV-vis) spectrophotometry, and ultraviolet photoelectron spectroscopy (UPS) analysis, we concluded that the luminescence type of the phosphors was bandgap luminescence. The addition of Cu+ significantly reduced the bandgap, produced defect levels, and promoted the electron transition between the bandgaps, which led to the strong red emission of PbBrOH:Cu under UV excitation. It is worth noting that the PbBrOH:Cu phosphor shows excellent environmental stability and excellent luminescence performance when it is used in organic solvent immersion experiments and manufacturing LED lamp beads. Therefore, the PbBrOH:Cu phosphor synthesized in this study is expected to be a strong candidate as the red phosphor component in white LEDs.


 Please wait while we load your content...
Please wait while we load your content...