Manipulating matrix stacking modes for ultralong-duration organic room-temperature phosphorescence in trace isomer doping systems†
Abstract
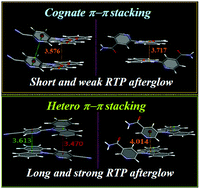
Derivatives prepared from commercial carbazole (CCZ) and carbazole synthesized in the laboratory (LCZ) have been found to display the same apparent compositions and structures but very different phosphorescence properties. Hence, CCZ derivative crystals should be treated as the doped phosphorescent material systems of trace carbazole isomer derivatives. In the current work, we obtained several typical stacking modes of a carbazole derivative matrix by changing the structure of the N-substituent. Cognate π–π stacking between carbazole rings was found to be very detrimental to room-temperature phosphorescence (RTP) lifetime and afterglow; instead, extensive hetero π–π stacking between carbazole and pyridine rings was favorable for ultralong-duration bright RTP. Moreover, two H-aggregates were observed to be highly fluorescent. This observation was particularly intriguing owing to the predominantly accepted view of H-aggregates and cognate π–π stacking being favorable for long-lived excited states and ultralong-duration and efficient phosphorescence. This work demonstrated that the influence of matrix stacking modes and intermolecular interactions on fluorescence and RTP are rather complex and related to the concrete molecular systems; and subtly manipulating the substituents can, remarkably, tune or alter light-emitting properties.


 Please wait while we load your content...
Please wait while we load your content...