Designing chemically selective liquid crystalline materials that respond to oxidizing gases†
Abstract
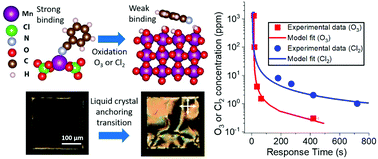
Computational methods can provide first-principles insights into the thermochemistry and kinetics of reactions at interfaces, but this capability has not been widely leveraged to design soft materials that respond selectively to chemical species. Here we address this opportunity by demonstrating the design of micrometer-thick liquid crystalline films supported on metal-perchlorate surfaces that exhibit selective orientational responses to targeted oxidizing gases. Initial electronic structure calculations predicted Mn2+, Co2+, and Ni2+ to be promising candidate surface binding sites that (1) coordinate with nitrile-containing mesogens to orient liquid crystal (LC) phases and (2) undergo redox-triggered reactions upon exposure to humid O3 leading to a change in the strength of binding of the nitrile group to the surface. These initial predictions were validated by experimental observations of orientational transitions of nitrile-containing LCs upon exposure to air containing parts-per-billion concentrations of O3. Additional first-principles calculations of reaction free energies of metal salts and oxidizing gases predicted that the same set of metal cations, if patterned on surfaces at distinct spatial locations, would provide LC responses that allow Cl2 and O3 to be distinguished while not responding to environmental oxidants such as O2 and NO2. Experimental results are provided to support this prediction, and X-ray diffraction measurements confirmed that the experimentally observed LC responses can be understood in terms of the relative thermodynamic driving force for formation of MnO2, CoOOH, or NiOOH from the corresponding metal cation binding sites in the presence of humid O3 and Cl2.


 Please wait while we load your content...
Please wait while we load your content...