Structure–property relationship in contrasting aggregation-induced enhancement/quenching of emission in rigid aromatic molecules†
Abstract
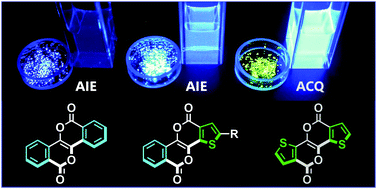
Aggregation-induced emission (AIE) has attracted considerable attention, and many types of molecules have been reported to show AIE. However, fully fused polycyclic π-systems remain unexplored as AIE luminogens because non-flexible structures are not suitable for AIE. Herein, we report a structure–AIE property relationship in a series of benzo- and/or thieno-fused pyrano[3,2-b]pyran-2,6-dione (PPD) derivatives. Although the π-conjugated skeleton is fully ring-fused and thus rigid, the benzo-fused PPDs exhibit AIE, while the bis-thieno-fused PPDs result in fluorescence quenching in the crystal. Notably, these contrasting behaviours do not depend on the substituents but on the peripheral fused aromatic rings, which does not follow the intuition that AIE is controlled by molecular flexibility. Vibrational analysis and investigation of photophysical rate constants revealed that in the benzo-fused PPDs the pyrone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations are coupled strongly with the nonradiative decay, enabling AIE activity of the rigid skeleton. This coupling is not pronounced in the bis-thieno-fused analogues, demonstrating that the rigid skeleton can be AIE active through vibronic control. Our work reveals a way to integrate the wide application area of AIE with the promising optoelectronic functions of polycyclic fused π-systems.
O stretching vibrations are coupled strongly with the nonradiative decay, enabling AIE activity of the rigid skeleton. This coupling is not pronounced in the bis-thieno-fused analogues, demonstrating that the rigid skeleton can be AIE active through vibronic control. Our work reveals a way to integrate the wide application area of AIE with the promising optoelectronic functions of polycyclic fused π-systems.


 Please wait while we load your content...
Please wait while we load your content...