Dual versus normal TADF of pyridines ornamented with multiple donor moieties and their performance in OLEDs†
Abstract
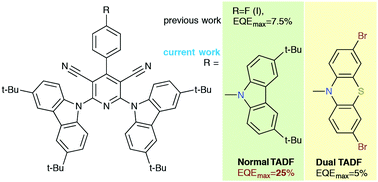
A procedure for post-functionalization by 3,6-di-tert-butyl-carbazole or 3,7-dibromophenothiazine of dicyanopyridines was developed. Pyridine rings in new pyridines exist in a twist-conformation in the solid state. Green and orange thermally activated delayed fluorescence (TADF) with close emission quantum yields in solid-state resulting from recombination of two different intramolecular charge transfer (ICT) states was observed for compounds post-functionalized by 3,6-di-tert-butyl-carbazole and 3,7-dibromophenothiazine moieties, respectively. Non-doped and doped organic light-emitting diodes exhibiting green and orange electroluminescence were developed using the synthesized compounds as normal/dual TADF emitters. The device based on 3,7-dibromophenothiazine-containing emitters exhibiting dual TADF showed low device life-times and a low maximum external efficiency of 3.1 (for the non-doped device) and 5% (for the doped device). Organic light-emitting diodes with 3,6-di-tert-butyl-carbazole-containing emitters exhibiting normal TADF showed relatively high device life-times and a high maximum external efficiency of 8.1 (for the non-doped device) and 25% (for the doped device). Because of the energy distribution between the two ICT states in dual-TADF emitters, ultra-long lifetime of fluorescence (up to milliseconds) was detected which results in exciton–exciton and exciton–polaron annihilations under electrical excitation and low stability and efficiency of the device.


 Please wait while we load your content...
Please wait while we load your content...