Synthesis of low-bandgap small molecules by extending the π-conjugation of the termini in quinoidal compounds†
Abstract
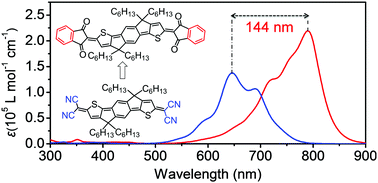
Low-bandgap π-conjugated small molecules have multiple applications. However, it is still a big challenge to develop such materials because of the scarcity of effective synthetic strategies. In this study, we adopt a feasible strategy, by extending the π-conjugation of the termini in quinoidal compounds, to construct low-bandgap π-conjugated small molecules. With this strategy, we synthesized four indandione-terminated quinoidal compounds, i.e., QIDT-H, QIDT-4F, QSiIDT-H and QSiIDT-4F. Owing to their closed-shell structures, which were confirmed by X-ray crystallographic analysis and 1H NMR spectroscopy, these compounds showed good air stability. Absorption spectroscopy revealed that the terminal benzene rings effectively extended the π-conjugation of these compounds and endowed them with a low bandgap. QIDT-H and QSiIDT-H showed similar bandgaps of ∼1.23 eV, which was smaller than that of a dicyanomethylene-terminated analogue (1.38 eV). Introducing fluorine atoms on the terminal benzene rings further reduced the bandgap, thus QIDT-4F and QSiIDT-4F showed very small bandgaps of 1.06 and 0.91 eV, respectively. These values are among the lowest reported to date for small molecules. The lowest unoccupied molecular orbital energy levels of these compounds were below −4.0 eV, giving them good electron-transport characteristics in organic thin-film transistors. Our findings demonstrate that extending the π-conjugation of the termini in quinoidal compounds is an effective strategy for developing low-bandgap π-conjugated small molecules.


 Please wait while we load your content...
Please wait while we load your content...