Synthesis of maleimide-based enediynes with cyclopropane moieties for enhanced cytotoxicity under normoxic and hypoxic conditions†
Abstract
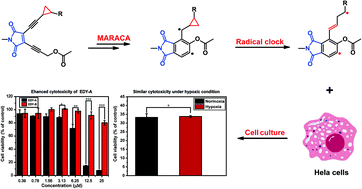
Myers-Saito cycloaromatization (MSC) is the working mechanism of many natural enediyne antibiotics with high antitumor potency. However, the presence of the equilibrium between diradical and zwitterionic intermediates in MSC severely hinders further improvement in cytotoxicity toward tumor cells. To this end, a series of maleimide-based enediynes with cyclopropane moieties were synthesized for enhanced cytotoxicity toward tumor cells. By taking advantage of radical clock reactions, the diradical intermediates generated from MSC would rearrange to new diradicals with much longer separation and weaker interactions between two radical centers. The computational study suggested a low energy barrier (4.4 kcal mol−1) for the radical rearrangement through the cyclopropane ring-opening process. Thermolysis experiments confirmed that this radical rearrangement results in the formation of a new diradical intermediate, followed by abstracting hydrogen atoms from 1,4-cyclohexadiene. Interestingly, the DNA cleavage ability and cytotoxicity of enediynes were significantly enhanced after the introduction of cyclopropane moieties. In addition, these maleimide-based enediynes exhibited a similar cytotoxicity under hypoxic conditions to that under normoxic conditions, which is beneficial for treating solid tumors where hypoxic environments frequently lead to deteriorated efficiency of many antitumor drugs. Docking studies indicated that the diradical intermediate was located between the minor groove of DNA with a binding energy of −7.40 kcal mol−1, which is in favor of intracellular DNA damage, and thereby inducing cell death via an apoptosis pathway as suggested by immunofluorescence analysis.


 Please wait while we load your content...
Please wait while we load your content...