Catalytic mechanism of spinel oxides for oxidative electrolyte decomposition in Mg rechargeable batteries†
Abstract
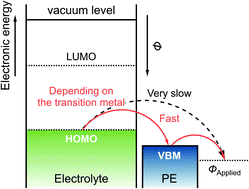
One of the primary drawbacks in the development of Mg rechargeable batteries is their low operating voltage. Although electrolytes with a wide potential window have been used for high-voltage Mg rechargeable batteries, these electrolytes undergo oxidative decomposition at the surface of the positive electrode active materials at relatively low potentials. Moreover, the overpotential and kinetics of oxidative electrolyte decomposition significantly depend on the transition metal ion in spinel oxides (e.g., MgMn2O4, MgFe2O4, or MgCo2O4) used as positive electrode active materials. Because the catalytic activities of spinel oxides for electrolyte decomposition are different, electrolyte decomposition can be effectively suppressed by using transition metal ions with high overpotential for electrolyte decomposition in target spinel oxides. However, the mechanism of the catalytic reaction has not yet been elucidated. Herein, we determined that the direct electron transfer from the electrolyte to the electrode was slow, whereas the electron transfer via the oxidation reaction of spinel oxides was fast. Furthermore, we used experimental data and calculations to demonstrate that the catalytic activity for oxidative electrolyte decomposition was correlated with the valence band maximum (VBM) of spinel oxides; that is, low VBMs were correlated with high overpotentials for oxidative electrolyte decomposition.


 Please wait while we load your content...
Please wait while we load your content...