Anodic hydrazine oxidation assisted hydrogen evolution over bimetallic RhIr mesoporous nanospheres†
Abstract
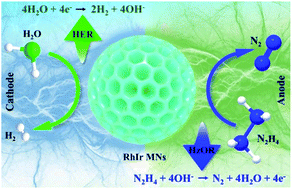
Replacing the sluggish anodic oxygen evolution reaction with the thermodynamically favorable hydrazine oxidation reaction (HzOR) is a powerful energy-saving approach for hydrogen production, and the efficiency of this process mainly relies on the design of a highly active catalyst. Herein, we propose a facile micelle-assisted reduction method for the development of uniform RhIr mesoporous nanospheres (RhIr MNs). Benefiting from the 3D mesoporous morphology and favorable electron structure, the RhIr MNs show superior electrocatalytic activity compared to the Rh MNs for both the hydrogen evolution reaction (HER) and HzOR in alkaline media. When the RhIr MNs are used as a bifunctional catalyst for the two-electrode overall hydrazine splitting, it only requires a low voltage of 0.13 V to afford 10 mA cm−2, much lower than that of the overall water splitting system. This proposed approach is universal for the rational design of efficient bifunctional Rh-based nanomaterials for hydrazine-assisted water splitting.


 Please wait while we load your content...
Please wait while we load your content...